Oncology Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional, Observational, Expanded Access), By Cancer Type, By Region, And Segment Forecasts, 2020 - 2027
- Report ID: GVR-4-68039-139-7
- Number of Pages: 120
- Format: Electronic (PDF)
- Historical Range: 2016 - 2018
- Industry:Healthcare
Report Overview
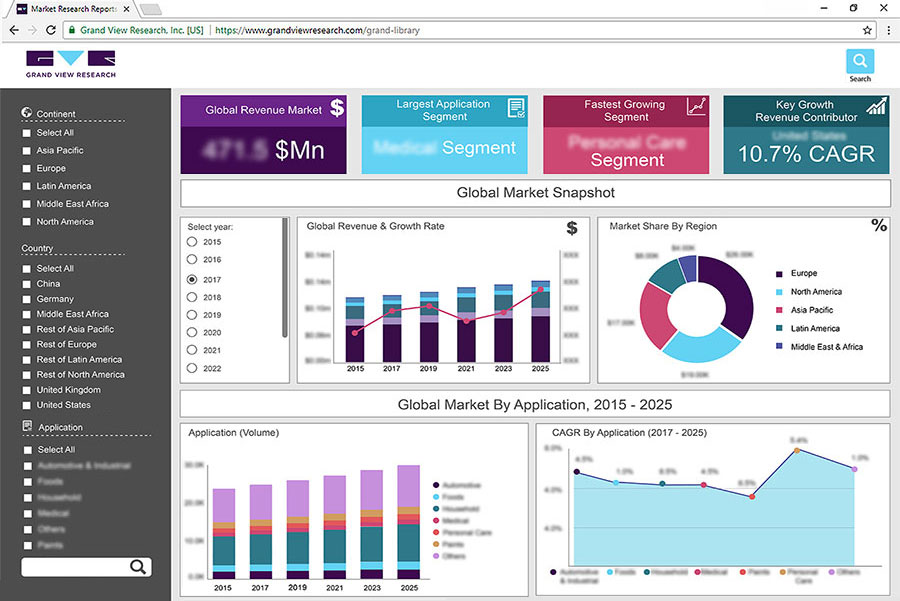
The global oncology clinical trials market size was valued at 10.8 billion in 2019 and is expected to grow at a compound annual growth rate (CAGR) of 5.4% from 2020 to 2027. The market is majorly driven by the increasing number of cancer cases, the need forpersonalized medicines,以及在研发活动上升cology. Besides, increasing innovation in the field of oncology and supportive government initiatives are also driving the market for oncology clinical trials. The number of people diagnosed with cancer is expected to increase multi-fold in the coming years. Although survivors have increased considerably owing to research in the oncology field, the number of cancer patients is expected to increase across the world. Lung cancer is the leading cause of cancer death with a projected 1.8 million people diagnosed each year in the world.

Pancreatic cancer has a poor prognosis with a survival of only 1 to 3 years. Around 13 individuals are diagnosed with gastrointestinal stromal tumor each day. Over 60,000 new cases of renal cell carcinoma are diagnosed every year in the U.S. Aforementioned factors are representing an increasing prevalence of cancer and a rising need for effective treatment against various types of cancers.
The research for treating cancer is moving forward at a rapid pace. A shift has been observed from chemotherapy protocols to molecularly targeted agents for immunotherapies in particular. Cancer continues to present novel challenges and opportunities for the intervention of pharmacologic. Consequently, the pharma industry continues to pursue oncology drug development at an unparalleled rate. The major trends include the evolution of clinical trials, a strong and growing pipeline of drug candidates, and sharp growth in available clinical data.
Since 1990, oncology was the industry’s largest focus on clinical drug development. This focus has continued to increase from the past several decades due to the scientific advancements in immunology, molecular biology, chemistry, cell biology, molecular, and genetics. The oncology trials continue to lead the biopharma efforts to develop new drugs. By the end of 2018, the number of compounds in the clinical development for oncology was more than twice the number of compounds in development for central nervous system disorders.
There has been an increasing number of cancer survivors living in the U.S., from 3.0 million in 1971 to 15.5 million in January 2016. Approximately 73.0% of the cancer survival gains are attributable to novel medicines. America’s biopharmaceutical research firms are developing effective and better-tolerated treatments to meet the needs of patients. An average of 85.0% of the oncology medicines in development are likely to be first-in-class. In 2018, there were 1,120 medicines and vaccines in development by America’s biopharmaceutical firms.
Phase Insights
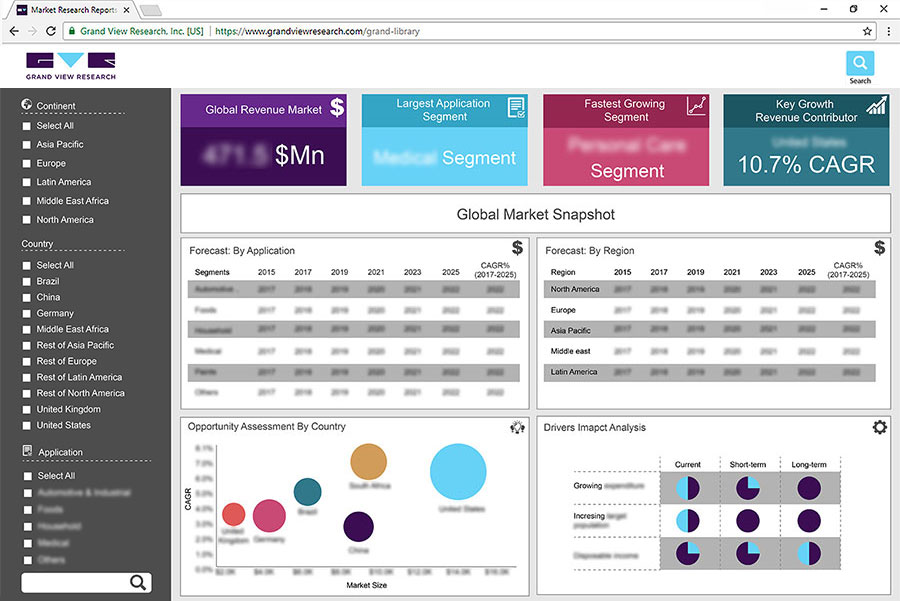
The phase II segment led the oncology clinical trials market and accounted for more than 45.0% share in 2019. The segment is also anticipated to maintain its position over the forecast period. This is attributed to the increasing number of studies in phase II. Besides, since 2010 there has been an improvement in the overall productivity of oncology clinical trials measured as success rates relative to the trial effort by 22%. Also, by Medicines Healthcare Products Regulatory Agency (MHRA), between 2011 and 2016, the application for phase II and phase III trials grew by 5.5%.
Besides, there has been an expansion in the drug pipeline for oncology in the late-stage development by 19.0% in 2018 alone. It reached 849 in 2018 from 711 in 2019 due to the growing number of targeted therapies in the pipeline. In 2018, 91.0% of the late-stage oncology pipeline was biologic therapies and targeted small molecules. Around 711 companies are working on 849 products, with the majority from emerging biopharmaceutical companies.
Cancer Type Insights
The leukemia segment led the market for oncology clinical trials and accounted for 23.8% of the revenue share in 2019. This is attributed to the increasing number of blood cancers around the globe. New cases of blood cancer are expected to account for 10.0% of total diagnosed cases of cancer in the U.S. The statistics indicate a growing trend of blood cancer across the U.S. and the trend is similar across the globe.
In 2017, leukemia was the most common cancer in children and accounted for about 30% of all childhood cancers. As per the American Society of Clinical Oncology (ASCO), multiple myeloma is the second most common blood cancer after non-Hodgkin lymphoma in the U.S. ASCO also projected that around 32,270 U.S. adults are likely to be diagnosed with multiple myeloma in 2020, thereby projecting the need to treat them.
Study Design Insights
The interventional design segment dominated the market for oncology clinical trials with a revenue share of 87.9% in 2019. It is one of the most prominent methods used in clinical trials. Interventional studies are categorized based on the intervention that is to be studied, which includes drug or biologic, behavioral, surgical procedures, and devices. There has been a significant rise in the number of interventional studies carried out over time.

The expanded access segment is anticipated to grow at a significant rate of 3.5% during the forecast period in the market for oncology clinical trials. Increasing innovation in clinical trial approaches is estimated to drive the segment over the forecast period. For instance, several drugs for oncology treatment are often administered to patients before the U.S. FDA approval and are considered as a part of expanded access trial.
Regional Insights
North America accounted for the largest revenue share of 55.0% in 2019 in the market for oncology clinical trials, and is expected to continue its dominance over the forecast period. This can be attributed to increasing R&D in this region, increasing adoption of new technologies in clinical research, as well as government support. For instance, the FDA has responded to the call with its adaptations in the way it reviews and approves new drugs for unmet needs of serious diseases, such as cancer. It has developed various guidelines, documents, and initiatives that aim at accelerating the development and approval of safe and effective drugs for cancer. It includes Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics; Clinical Trial Imaging Endpoint Process Standards; the FDA Accelerated Approval Program; Breakthrough Therapy; Accelerated Approval; Priority Review; and Fast Track.
Europe followed North America in terms of market share and contributed to 24.2% of the revenue share in 2019 in the market for oncology clinical trials. This is attributed to the favorable government initiatives being taken in this region. For instance, in 2015, the EMA released guidelines to accelerate the assessment of eligible medicines. In 2016, it launched the Priority Medicines (PRIME) scheme to classify those products at an early stage of development that have the potential to address unmet medical needs.
In Asia Pacific, the market for oncology clinical trials is anticipated to witness the fastest CAGR of 7.4% during the forecast period owing to the increasing availability of a large patient pool enabling easy recruitment of candidates. As per Asia Pacific’s largest expertized biotech CRO “Novotech”, the region has become a preferred destination for clinical studies due to the lower trial density, and a large number of active investigators. Many biotechnology firms seeking oncology CRO services are increasingly turning to this region.
Key Companies and Market Share Insights
肿瘤临床试验是高度的市场competitive in nature. A significant factor affecting the competitive nature of the market is the quick adoption of advanced technology for improved healthcare. Also, to retain share and expand the product portfolio, major players are often involved in mergers and acquisitions along with new product launches and collaborations. For instance, in September 2019, IQVIA announced its collaboration with Cancer Researchers to advance the use of real-world evidence and expand clinical research in oncology. In June 2018, Acurian and Synexus, a part of PPD, launched SynexusPlus. SynexusPlus is a site solution for patient enrollment in clinical studies. This initiative is anticipated to improve clinical trial productivity. Some of the prominent players in the oncology clinical trials market include:
PAREXEL International Corporation
PRA Health Sciences
Syneos Health
Medpace
Novotech
Pivotal
Oncology Clinical Trials Market Report Scope
Report Attribute |
Details |
Market Size value in 2020 |
USD 11.5 billion |
Revenue forecast in 2027 |
USD 16.6 billion |
Growth Rate |
CAGR of 5.4% from 2020 to 2027 |
Base year for estimation |
2019 |
Historical data |
2016 - 2018 |
Forecast period |
2020 - 2027 |
Quantitative units |
Revenue in USD million and CAGR from 2020 to 2027 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Phase, study design, cancer type, region |
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; India; Japan; China; Thailand; South Korea; Brazil; Mexico; Argentina; Colombia; South Africa; Saudi Arabia; UAE |
Report coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key companies profiled |
IQVIA; PAREXEL International Corporation; Pharmaceutical Product Development, LLC; Charles River Laboratory; ICON Plc; PRA Health Sciences; Syneos Health; Medpace; Novotech; Pivotal |
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
革命制度党cing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2027. For the purpose of this study, Grand View Research has segmented the global oncology clinical trials market report on the basis of phase, study design, cancer type, and region:
Phase Outlook (Revenue, USD Million, 2016 - 2027)
Phase I
Phase II
Phase III
Phase IV
Study Design Outlook (Revenue, USD Million, 2016 - 2027)
Interventional
Observational
Expanded Access
Cancer Type Outlook (Revenue, USD Million, 2016 - 2027)
Lung cancer
Breast cancer
Thyroid cancer
Leukemia
Liver cancer
Skin cancer
Lymphoma
Pancreatic cancer
Prostate cancer
Colon & rectal cancer
Urinary system cancer
Other cancer
Regional Outlook (Revenue, USD Million, 2016 - 2027)
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Asia Pacific
India
Japan
China
Thailand
South Korea
Latin America
Brazil
Mexico
Argentina
Colombia
Middle East & Africa
South Africa
Saudi Arabia
UAE
Frequently Asked Questions About This Report
b.The global oncology clinical trials market size was estimated at USD 10.8 billion in 2019 and is expected to reach USD 11.5 billion in 2020.
b.The global oncology clinical trials market is expected to grow at a compound annual growth rate of 5.4% from 2020 to 2027 to reach USD 16.6 billion by 2027.
b.North America dominated the oncology clinical trials market with a share of 55.0% in 2019. This can be attributed to increasing R&D in this region, increasing the adoption of new technologies in clinical research as well as government support.
b.Some key players operating in the oncology clinical trials market include IQVIA, PAREXEL International Corporation, Pharmaceutical Product Development, LLC; PAREXEL International Corporation, and Charles River Laboratory.
b.Key factors that are driving the oncology clinical trials market growth include the increasing number of cancer cases, the need for personalized medicines, as well as the rise in the R&D activities for oncology. Besides, increasing innovation in the field of oncology & supportive government initiatives is also driving the market growth.